In our recent webinar, “Improving the safety, visibility & transparency of your supply chain during a crisis to sustain customer confidence,” we joined with ComplianceQuest to demonstrate how seamlessly integrated manufacturing, compliance and quality on a single cloud platform helps medical device manufacturers improve operations and enjoy enhanced growth.
Focus on Medical Device Industry Challenges
In the first part of the webinar, our partner ComplianceQuest reviewed some of specific challenges facing medical device companies, including:
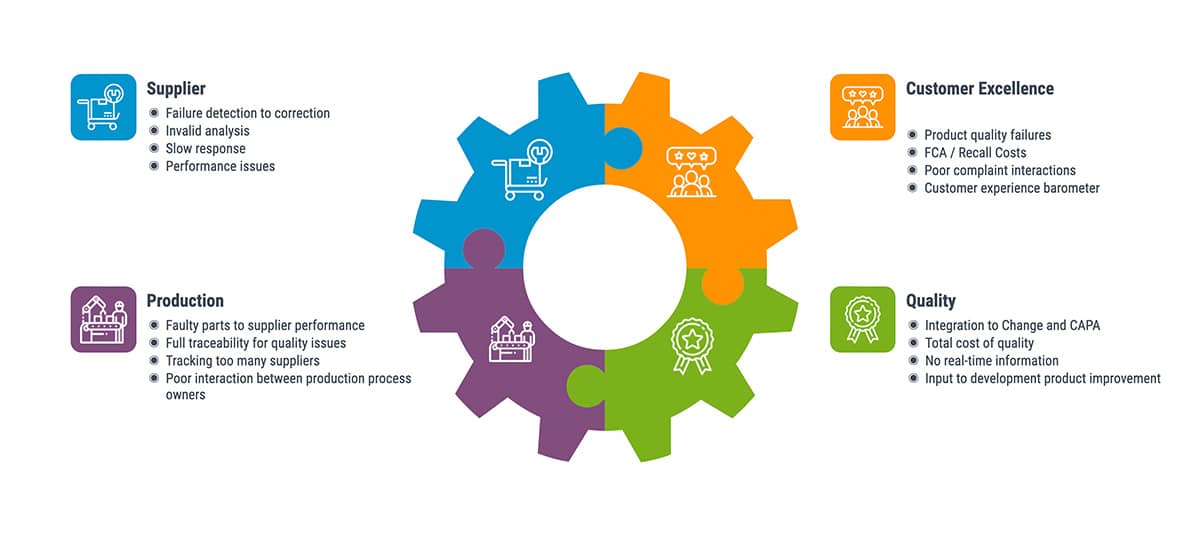
- How complex interactions between suppliers, production, quality management and customers create the need for a single, integrated system of record.
- How information silos create problems arising from manual transfers of information, isolated systems of record and no common system of engagement, and how a common system of data provides a single “truth” for everyone.
- How the Covid-19 pandemic has disrupted supply chains and revealed problems in the medical device industry.
- Why handling complaints is an essential part of quality and compliance for medical device companies.
The webinar speakers also addressed recalls, which is an important topic in the med device industryDid you know that more medical devices were recalled in 2019 than there were in each of the previous four years? Did you also know that 55% of medical device recalls are nationwide and 36% of recalls had international distribution?
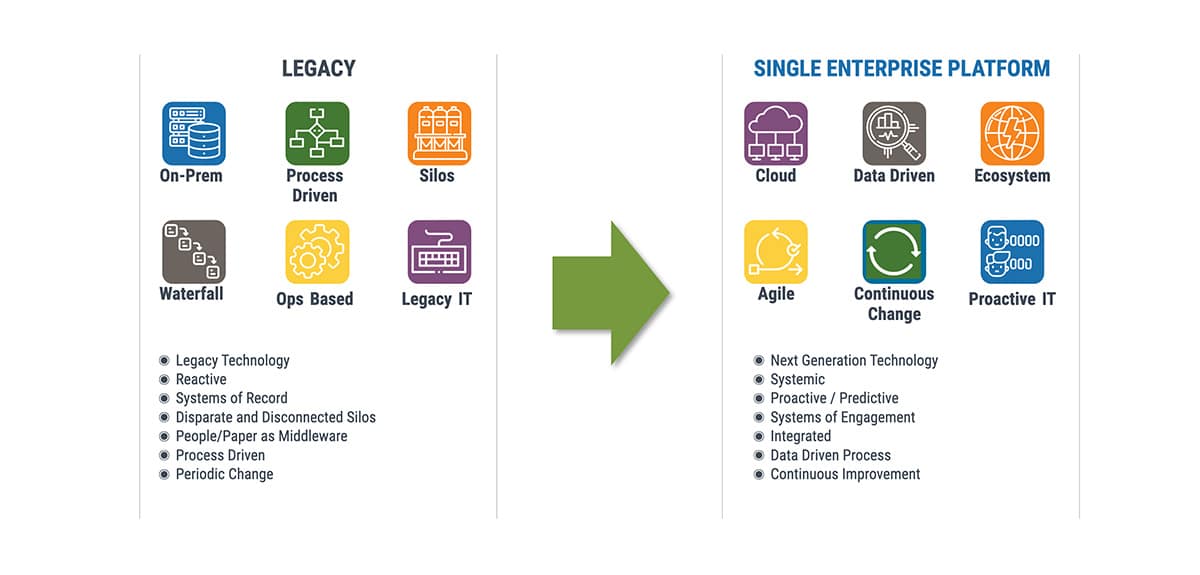
Imagine how costly and unmanageable it can be to identify the root cause of a recall if you cannot track products or investigate customer complaints without visibility into the supply chain. Legacy, on-premise technology, multiple systems of record and manual, paper- and people-driven processes simply are not equipped to allow data-driven tracking and tracing across an entire enterprise.
Rootstock Cloud ERP and ComplianceQuest EQMS on the Salesforce cloud platform is an integrated solution that is designed to help medical device manufacturers resolve such problems quickly and easily.
Focus on Rootstock Cloud ERP and ComplianceQuest EQMS
The second part of the webinar demonstrated how Rootstock Cloud ERP and Compliance EQMS work together to provide a truly integrated solution with a single customer engagement record.
Agility in the Age of COVID-19
As part of the Salesforce cloud platform, Rootstock Cloud ERP and ComplianceQuest EQMS gives customers more than just the ability to track and trace product history to resolve customer complaints. The Salesforce platform itself has enormous flexibility that has allowed some manufacturers to respond quickly to the dramatic changes wrought by the COVID-19 pandemic. For example, Matouk, a manufacturer of luxury linens, was able to react to the sudden drop in demand for their products quickly shifting to the production of face masks when the shortage of PPE became evident.
Another Rootstock customer, Unionwear, a New Jersey-based manufacturer of made-in-the-US apparel and accessories, shifted to making face shields and gowns when their orders all dried up due to the pandemic.
In both cases, the Salesforce platform provided the technology that enabled the shift in production. Both companies were able to quickly design, produce and ship new PPE products to customers like hospitals who were in desperate need of them. That’s agile manufacturing in a nutshell.
How Our Solution Makes It Easy to Handle Customer Complaints
After reviewing the advantages of our solution, the webinar speakers demonstrated how we help you handle a typical customer complaint, from viewing the high-level complaint record all the way down to tracking the details of the product’s history to locate the root cause of the problem and initiate a solution.
In our example, we investigated the failure of a drug-coated stent. We were able to quickly find where this product has been, who may have bought it and what orders it was associated with to help us determine if there might be a larger problem. We used Rootstock’s global search to quickly find the serial number of the stent, and then quickly see where that serial number has been used. Was the stent part of a specific lot and if so, what is the status of the other stents in that lot?
We were able to quickly view the order history of the product and determine if there was a problem with the drug coating. If the coating was not the source of the problem, was it the packaging? If it was the packaging, our integrated solution lets you identify the problem quickly and initiate an engineering change order to correct it.
As you’ll see in the webinar, the ability to track and trace a product or product component all the way through the system is critical to medical device companies. This is a significant advantage that our integrated solution provides and will help you overcome the problem of information silos and manual, spreadsheet-driven processes, as well as legacy systems that were never designed for this kind of process.
More Resources for Medical Device Manufacturers
If you want to learn more about to solve the challenge of growing compliance requirements and increasing supply chain complexity in the wake of the pandemic crisis, watch for our upcoming podcast on the subject, available soon on our Podcast page. You’ll hear how your medical device company can take a more comprehensive approach to manufacturing, compliance, quality and service by putting the right systems and best practices in place.